Exceptional painting - exceptional lead binding
Micro and macro X-ray analysis detects lead formate in Rembrandt's Night Watch
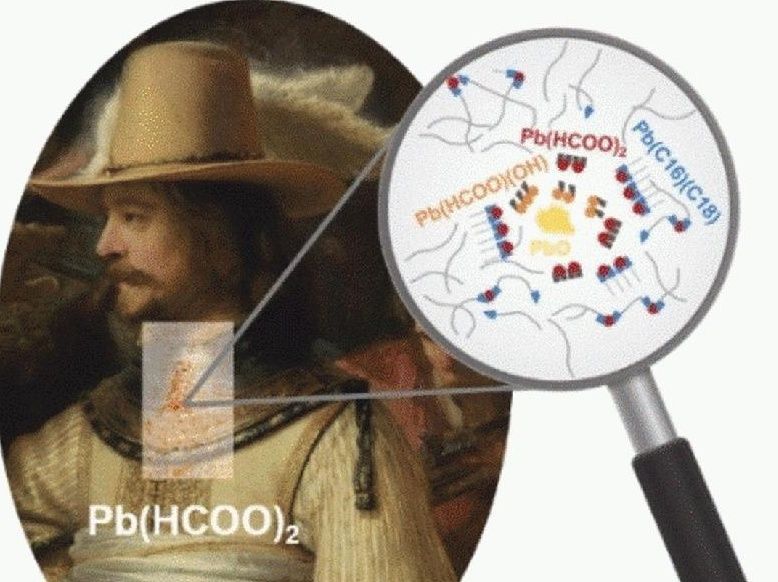
Rembrandt van Rijn was one of the most important Dutch painters of the 17th century. Probably his most famous painting is The Night Watch from 1642, which can be admired today in the Rijksmuseum in Amsterdam. An international team has now identified lead formate in several areas of the Night Watch, a very unusual compound for paintings. The findings, presented in the journal Angewandte Chemie, provide new clues regarding Rembrandt's painting practices and the reactivity of lead drying agents in oil matrices of historical paintings.
Exceptional painting - exceptional lead binding
(c) Wiley-VCH
"Operation Night Watch" is an extensive research and conservation project on Rembrandt's Night Watch, involving close collaboration between conservators, art historians, and other scientists. In this framework, composition and material distribution were determined by large-area X-ray powder diffraction, and in parallel, micro-X-ray methods with synchrotron radiation as well as infrared microscopy were performed on tiny samples. The team from the Rijksmuseum as well as the University of Amsterdam, the CNRS, the European Synchrotron Radiation Facility in France, and the University of Antwerp, among others, succeeded in identifying and mapping various lead compounds in Rembrandt's paint layers.
Lead pigments were widely used by Rembrandt. The most common was lead white, a mixture of the lead carbonates hydrocerussite Pb3(CO3)2(OH)2 and cerussite PbCO3. In addition, lead occurs in other pigments and compounds formed from them. In contrast, another discovery made by the team led by Victor Gonzalez, Ida Fazlic and Marine Cotte was very unusual: lead(II) formate Pb(HCOO)2 - a compound that had never been found before in historical oil paintings. Lead formate, the lead salt of formic acid, has been identified in several areas of the vigil-in some cases along with plumbonacrite, Pb5(CO3)3O(OH)2, another rare lead compound.
To explore the chemical origin of the lead formate, the team made model paint layers using ancient formulations. For example, an oil drying agent was made by heating linseed oil, the most common binder for paints at the time, with lead oxide PbO. Lead oxide is a metal-containing drying agent that cures paints more quickly.
As it turned out, PbO can react in oil paints to form lead formate. Even though no crystalline PbO was detected in the vigil, the results support the hypothesis that an oil containing such a lead drying agent was used. But other hypotheses must also be considered. Past conservation work on the Night Watch, particularly the possible application of an oil-based varnish in the 18th century, may have favored the formation of lead formate on the painting. The team is now investigating the kinetics of lead formate formation and its stability in oil paints.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Most read news
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
From now on, don't miss a thing: Our newsletter for the chemical industry, analytics, lab technology and process engineering brings you up to date every Tuesday and Thursday. The latest industry news, product highlights and innovations - compact and easy to understand in your inbox. Researched by us so you don't have to.

























































